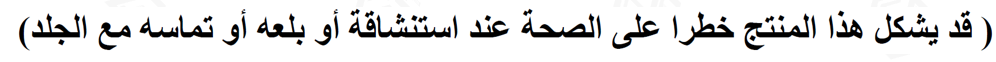background:
The vaping industry first adopted the 2019 Principles for Tobacco and Nicotine Products, Including Electronic Products, of 2019 Issued by the Jordan Food and Drug Administration based on the Council of Ministers Decision No. 4569 of May 8, 2019, and Article 7(l) of Jordan Food and Drug Administration Law No. 41 of 2008”), which regulates product restrictions, labelling, advertising and health warnings the rule of. Another standard "Requirements for the Display, Sale, and Storage of Electronic Vaping Products, Electronic liquid Products, and Electronically Heated Tobacco Products" stipulates Advertising, product display and retail restrictions. Trade in tobacco and nicotine vaping products, e-liquid products, and electronic heated tobacco products must be approved and registered by JDFA and a sales license obtained from JDFA. JDFA is the regulatory agency Jordan Food and Drug Administration.
Scope of application:
-
Vaporizers and e-liquids, with or without nicotine, and containers designed to be refilled;
-
Electronic heat-not-burn tobacco products (processed or unprocessed);
-
Other similar products included in the above concept;
e-liquid requirements:
-
The concentration of free nicotine in e-liquid should not exceed 20mg/mL;
-
The concentration of free nicotine salts in e-liquid should not exceed 25mg/mL;
-
The volume of cartridges or disposable e-liquids cannot exceed 2ml;
-
The volume of bottled e-juice cannot exceed 10ml;
-
The nicotine and solvents used in the e-liquid must be of high pharmaceutical-grade purity and meet the requirements of the European Pharmacopoeia; except for nicotine, heated or unheated forms will not pose a risk to human health;
-
The microbial limit of nicotine used in e-liquid cannot exceed the provisions of the European Pharmacopoeia for non-sterile inhalation products;
-
The seasoning materials and additives used must comply with food grade standards or ingredients permitted for inhalation equipment by "Jordanian standard 94 and its amendments";
-
Food grade glycerin, 1-2 propylene glycol, 1-3 butanediol and triethylene glycol (purity of at least 99.5%) can be added to the tobacco oil. These ingredients need to meet the requirements of pharmaceutical grade and European Pharmacopoeia;
-
When heated or unheated, and used at a certain concentration level according to appropriate instructions, any material other than nicotine in cigarette oil shall not cause health harm to users;
-
Study on the stability of the product shelf life marked on the product package;
-
The following substances shall not be contained in the smoke oil and its emissions:
-
Vitamins or other additives give people the impression that the product is beneficial to health or reduces health risks;
-
Caffeine, taurine or other additives and stimulants related to stimulating performance and vitality;
-
Colorants;
-
Carcinogens, mutagens, or toxins produced in non combustion or combustion forms;
-
Substances listed as prohibited by law, such as narcotics, hallucinogens, tranquilizers or stimulants;
-
Long chain preservatives of ethylene glycol, diethylene glycol, formaldehyde, acetaldehyde, acrolein, acetone, acetylpropionyl and diacetyl, p-hydroxybenzoate;
-
Respiratory hypersensitivity source;
-
Heavy metals such as lead, cadmium, mercury, chromium, nickel, iron, arsenic and tin;
-
The microbial limit should not exceed the European pharmacopoeia for non sterile inhaled products;
-
Any substance proved to be harmful to health or considered by the committee to be prohibited in the product.
Basic requirements for electronic equipment and components
-
The electronic atomizer container must be food grade and not easy to crack, damage or crush;
-
It must be ensured that the smoke oil in the atomizer cannot leak into the mouth during suction;
-
Other components of the product, such as paper and packaging, shall not contain spices that change the odor, taste or emission density, and shall not contain tobacco or nicotine;
-
Electronic atomizers and refilled containers must be child proof and tamper proof to avoid safety risks to children; Prevent damage and leakage, and there is a mechanism of refilling without leakage;
-
US FDA CFR 21 certificate and CE certification.
Packaging requirements:
-
Product name and trade name;
-
The quantity of products in each package;
-
The composition and concentration of the product and the concentration of nicotine (must be clearly stated on the package);
-
Shelf life or packaging date of the product (stability study, if any);
-
batch number;
-
Product ingredients;
-
Country of origin, manufacturer or packer;
-
The phrase "sold in Jordan";
-
Nicotine health warning (must be in Arabic or English), covering 30% of the main display area, with English at the bottom of the front and Arabic at the bottom of the back;
(Contains nicotine. Nicotine causes severe addictions, increased heart rate and high blood pressure. Nicotine is harmful to pregnant and nursing women and people suffering from asthma)
-
Health warnings are printed on the package and any outer package and are completely visible; It cannot be erased or removed, including partially or completely hidden or completely covered, whether it is price tag, safety function, wrapping paper or others;
-
It is forbidden to sell warnings (Arabic and English) and icons to people under the age of 19, and the size of the icon shall not be less than 1cm*1cm;
(It is prohibited to sell and consume this product by individuals under the age of 19, and it is not recommended for non-smokers.)
-
The following warnings (Arabic and English) need to be added to products containing tobacco oil;
(This product may pose a health hazard when inhaled, swallowed or get in contact with the skin)
-
The information must be clear and readable, and the text color must be different or different from the background color, and must not overlap;
-
The outer cellophane wrapping paper shall not have any statement, name, image, mark, conformity or information.
-
The consumption of electronic heated tobacco and nicotine products should not be encouraged by creating a false impression of their characteristics or health effects or risks or their releases, such as:
-
Printing vouchers, discounts or free distribution concessions or any other similar concessions that may give consumers the impression of economic benefits;
-
Claim that they have vitality, activity, therapeutic, revitalizing, natural or organic properties;
-
Claim that they have other health or lifestyle benefits;
-
Claim that they are like food;
-
Claim that they are less harmful to the environment;
-
The phrase "causing small harm / less harm" appears
-
Not marked with taste, smell or other additives (except seasoning);
-
Do not use any name, symbol, mark, picture or declaration order that violates the public interest;
-
Description cards or data shall not be used to describe or provide the products and their accessories, names, forms or symbols, or any packaging features that may cause false, misleading or promotional impressions related to the unapproved suggestive slogans inside or outside.
-
Each product package must contain a manual containing the following information, printed in Arabic and English:
-
Instructions for the use and storage of the product, including instructions that non-smokers under the age of 19 are not recommended to use the product;
-
Product taboos and warnings for certain consumer groups;
-
Addiction and toxicity;
-
Potential hazards.
Registration requirements for electronic atomization equipment:
-
The letter from the importer requesting registration, including the name, type, manufacturer and country of origin of the commodity to be registered.
-
The valid business registration certificate issued by the Ministry of industry and trade must include the right to import / tobacco trade.
-
Valid business license of importer.
-
Valid certificate.
4.1US FDA CFR 21 certificate.
4.2. The CE certificate issued by the EU national authority meets the following requirements:
-
RoHS Directive (2011/65/eu): restrict the use of certain hazardous substances in electrical and electronic equipment.
-
LVD (2014/35/eu): Low Voltage Directive
-
EMC :Electromagnetic Compatibility Directive
-
Product catalog or label file containing product instructions, including opening and closing mechanisms, refilling, storage methods, safety instructions, etc
-
Equipment specifications or safety data certificates (SDS, MSDS or PSDs) include components, uses, contraindications, warnings, etc.
-
Toxicity study of combustion and unburned components and emissions
-
Pictures of the outer and inner packaging of the product.
-
Instructions (if any).
-
Safety test reports issued by scientific institutions or laboratories recognized by jfda - drop test, leakage test, interface temperature test, electromagnetic efficiency
-
A statement that the manufacturer and importer bear full responsibility for the safety and quality of the product when it is used in normal and predictable ways after it is put on the market.
-
There must be a barcode on the outer package of the equipment.
-
The equipment and its accessories must have serial numbers.
-
If any changes are made to the materials previously reviewed and approved, the application must be resubmitted for re review and approval.
Registration requirements for tobacco oil (taste is registered separately):
-
The letter from the importer requesting registration, including the name, type, manufacturer and country of origin of the commodity to be registered.
-
A valid importer business register issued by the Ministry of industry and trade, which includes the purpose of importing / trading electronic atomizers.
-
The original of the certificate of free sale in the country of origin issued by the relevant official authority, showing:
-
The name and trademark of the product to be approved.
-
The name of the manufacturing and / or packaging company.
-
The manufacturer has obtained the license to produce tobacco oil and is subject to health monitoring.
-
The product conforms to the production specification.
-
GMP or evidence proving its compliance with internationally recognized manufacturing quality management system.
-
The ingredient certificate issued by the manufacturer shows all ingredients in the product in descending order of weight, including the percentage of nicotine and the percentage of purity.
-
The analysis certificate issued by the manufacturer shows the percentage of nicotine according to the allowable limit.
-
A certificate issued by the manufacturer stating that the container is food grade and can be safely used for filling tobacco oil.
-
Toxicological study of product components and their emissions.
-
Product samples.
-
Copy of information card (outer package and instructions).
-
Electronic copy of the above documents
-
If any changes are made to the materials previously reviewed and approved, the application must be resubmitted for re review and approval.
Test requirements:
Tobacco oil
-
Toxicological analysis report.
-
Release test report and data;
-
The composition analysis of tobacco oil includes nicotine content;
Equipment
-
IEC 60335-1Test report;
-
IEC 62321Test report;
-
EMCTest report;
-
FDATesting of food contact materials.